WARNING
SEVERE MYELOSUPPRESSION and CARCINOGENICITY
- TEPYLUTE may cause severe marrow suppression, and high doses may cause marrow ablation with resulting infection or bleeding. Monitor hematologic laboratory parameters. [see WARNINGS AND PRECAUTIONS].
- TEPYLUTE should be considered potentially carcinogenic in humans [see WARNINGS AND PRECAUTIONS].
Description for Tepylute
TEPYLUTE injection contains thiotepa, an alkylating drug. The chemical name for thiotepa is Tris(1-aziridinyl)phosphine sulfide. Thiotepa has the following structural formula:
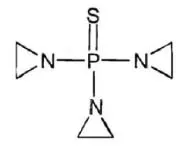 |
Thiotepa has the molecular formula C6H12N3PS, and a molecular weight of 189.23, and it appears as fine, white crystalline flakes, with a melting range of 52°C to 57°C. It is soluble in water and organic solvents. Thiotepa is unstable in acid medium.
TEPYLUTE (thiotepa) injection is supplied as a sterile solution for intravenous use after dilution. When diluted in water, the resulting solution has a pH of approximately 5.5 to 7.5.
TEPYLUTE injection is a 10 mg/mL solution available as:
- 15 mg/1.5 mL strength vial contains 15 mg thiotepa and 1.7 g of polyethylene glycol 400
Uses for Tepylute
Adenocarcinoma Of The Breast Or Ovary
TEPYLUTE is indicated for treatment of adenocarcinoma of the breast or ovary.
Dosage for Tepylute
Recommended Dosage
Adenocarcinoma Of The Breast Or Ovary
The recommended dose of TEPYLUTE for treatment of adenocarcinoma of the breast or ovary is 0.3 mg/kg to 0.4 mg/kg intravenously. Doses should be given at 1 to 4 week intervals. Initially the higher dose in the given range is commonly administered. The maintenance dose should be adjusted weekly on the basis of pretreatment control blood counts and subsequent blood counts.
Maintenance doses should not be administered more frequently than weekly.
Preparation Instructions
TEPYLUTE is a hazardous drug. Follow applicable special handling and disposal procedures1.
Use aseptic technique to prepare TEPYLUTE. Each vial is intended for single-dose only. Discard any unused portion left in the vial.
Dilution In The Infusion Bag
- Remove vial from refrigerated conditions 1 hour prior to dilution.
- Dilute the solution in an appropriate volume of 0.9% Sodium Chloride Injection to obtain a final TEPYLUTE concentration between 0.5 mg/mL and 1 mg/mL. Discard unused portion. Use a 16G needle with a Luer-lock syringe to dilute the solution.
Use the diluted TEPYLUTE infusion solution immediately. If the solution is not used immediately, store refrigerated at 2°C to 8°C (36°F to 46°F) for up to 24 hours, or at room temperature 25°C (77°F) for up to 4 hours.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Use TEPYLUTE diluted solutions only if free of visible particulate matter. Filter using a 0.2 micron filter prior to administration. Filtering does not alter solution potency.
HOW SUPPLIED
Dosage Forms And Strengths
- Injection: 15 mg/1.5 mL (10 mg/mL) of thiotepa in a clear, colorless or almost colorless solution in single-dose vial.
TEPYLUTE injection is supplied as a clear, colorless or almost colorless solution in a single-dose amber glass vial in a carton. The vial stopper is not made with natural rubber latex.
TEPYLUTE 15 mg/1.5 mL (10 mg/mL)
Each vial contains 15 mg thiotepa (NDC 81927-105-15).
Storage And Handling
TEPYLUTE injection vials must be stored and transported refrigerated at 2°C to 8°C (36°F to 46°F).
Do not freeze. TEPYLUTE injection is a hazardous drug. Follow applicable special handling and disposal procedures1.
REFERENCES
1. OSHA Hazardous Drugs. OSHA. [Accessed from http://www.osha.gov/SLTC/hazardousdrugs/index.html].
Manufactured by: AqVida GmbH, Dassow, Germany. Revised: Jun 2024.
Side Effects for Tepylute
The following clinically significant adverse reactions are described elsewhere in other sections of the label:
- Myelosuppression [see WARNINGS AND PRECAUTIONS]
- Infection [see WARNINGS AND PRECAUTIONS]
- Hypersensitivity [see WARNINGS AND PRECAUTIONS]
- Cutaneous Toxicity [see WARNINGS AND PRECAUTIONS]
- Hepatic Veno-Occlusive Disease [see WARNINGS AND PRECAUTIONS]
- Central Nervous System Toxicity [see WARNINGS AND PRECAUTIONS]
- Carcinogenicity [see WARNINGS AND PRECAUTIONS]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions With Treatment Of Adenocarcinoma Of The Breast And Adenocarcinoma Of The Ovary
Gastrointestinal: Nausea, vomiting, abdominal pain, anorexia.
General: Fatigue, weakness. Febrile reaction and discharge from a subcutaneous lesion may occur as the result of breakdown of tumor tissue.
Hypersensitivity Reactions: Allergic reactions -rash, urticaria, laryngeal edema, asthma, anaphylactic shock, wheezing.
Local Reactions: Contact dermatitis, pain at the injection site.
Neurologic: Dizziness, headache, blurred vision.
Renal: Dysuria, urinary retention, chemical cystitis or hemorrhagic cystitis.
Reproductive: Amenorrhea, interference with spermatogenesis.
Respiratory: Prolonged apnea has been reported when succinylcholine was administered prior to surgery, following combined use of thiotepa and other anticancer agents. It was theorized that this was caused by decrease of pseudocholinesterase activity caused by the anticancer drugs.
Skin: Dermatitis, alopecia. Skin depigmentation has been reported following topical use.
Special Senses: Conjunctivitis.
Postmarketing Experience
The following adverse reactions have been identified during post approval use of thiotepa. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders: Febrile bone marrow aplasia.
Cardiac disorders: Bradycardia, cardiac failure congestive, cardio-respiratory arrest, pericardial effusion, pericarditis, right ventricular hypertrophy.
Congenital, familial and genetic disorders: Aplasia.
Ear and labyrinth disorders: Deafness.
Eye disorders: Blindness, eyelid ptosis, papilledema, strabismus.
Gastrointestinal disorders: Ascites, dysphagia, enterocolitis, gastritis, palatal disorder.
General disorders and administration site conditions: Device related infection, gait disturbance, malaise, multi-organ failure, pain.
Hepatobiliary disorders: Hepatomegaly.
Immune system disorders: Bone marrow transplant rejection, immunosuppression.
Infections and infestations: Acute sinusitis, bronchopulmonary aspergillosis, candida sepsis, enterococcal infection, Epstein-Barr virus infection, Escherichia sepsis, Fusarium infection, gastroenteritis, infection, lower respiratory tract infection fungal, lower respiratory tract infection viral, parainfluenza virus infection, Pneumonia legionella, relapsing fever, respiratory tract infection, sepsis, septic shock, Staphylococcal bacteremia, Staphylococcal infection, systemic candida, urinary tract infection.
Injury, poisoning and procedural complications: Refractoriness to platelet transfusion, subdural hematoma.
Investigations: Coagulation test abnormal, hemoglobin decreased, Klebsiella test positive, nuclear magnetic resonance imaging brain abnormal, transaminases increased, weight increased.
Metabolism and nutrition disorders: Hyponatremia.
Neoplasms benign, malignant and unspecified (incl. cysts and polyps): Breast cancer metastatic, central nervous system lymphoma, leukemia recurrent, lymphoma, malignant neoplasm progression, metastatic neoplasm, post-transplant lymphoproliferative disorder.
Nervous system disorders: Aphasia, brain injury, bulbar palsy, central nervous system lesion, cerebral microangiopathy, cerebral ventricle dilatation, cerebrovascular accident, cognitive disorder, convulsion, coordination abnormal, encephalitis, encephalopathy, hemiplegia, hypotonia, leukoencephalopathy, memory impairment, motor dysfunction, neurotoxicity, quadriparesis, speech disorder, tremor, VIIth nerve paralysis, white matter lesion.
Psychiatric disorders: Delirium, depression, disorientation, suicidal ideation.
Renal and urinary disorders: Renal failure, nephropathy toxic.
Respiratory, thoracic and mediastinal disorders: Acute respiratory distress, aspiration, dyspnea exertional, interstitial lung disease, lung disorder, pneumonitis, pulmonary arteriopathy, pulmonary sepsis, pulmonary veno-occlusive disease, respiratory distress, respiratory failure, pulmonary hypertension.
Skin and subcutaneous tissue disorders: Stevens-Johnson syndrome and toxic epidermal necrolysis.
Vascular disorders: Capillary leak syndrome.
Drug Interactions for Tepylute
Effect Of Cytochrome CYP3A Inhibitors And Inducers
In vitro studies suggest that thiotepa is metabolized by CYP3A4 and CYP2B6 to its active metabolite TEPA. Avoid coadministration of strong CYP3A4 inhibitors (e.g., itraconazole, clarithromycin, ritonavir) and strong CYP3A4 inducers (e.g., rifampin, phenytoin) with thiotepa due to the potential effects on efficacy and toxicity [see CLINICAL PHARMACOLOGY]. Consider alternative medications with no or minimal potential to inhibit or induce CYP3A4. If concomitant use of strong CYP3A4 modulators cannot be avoided, closely monitor for adverse drug reactions.
Effect Of TEPYLUTE On Cytochrome CYP2B6 Substrates
In vitro studies suggest that thiotepa inhibits CYP2B6. Thiotepa may increase the exposure of drugs that are substrates of CYP2B6 in patients; however, the clinical relevance of this in vitro interaction is unknown [see CLINICAL PHARMACOLOGY]. The administration of thiotepa with cyclophosphamide in patients reduces the conversion of cyclophosphamide to the active metabolite, 4-hydroxycyclophosphamide; the effect appears sequence dependent with a greater reduction in the conversion to 4-hydroxycyclophosphamide when thiotepa is administered 1.5 hours prior to the intravenous administration of cyclophosphamide compared to administration of thiotepa after intravenous cyclophosphamide [see CLINICAL PHARMACOLOGY]. The reduction in 4-hydroxycyclophosphamide levels may potentially reduce efficacy of cyclophosphamide treatment.
Warnings for Tepylute
Included as part of the "PRECAUTIONS" Section
Precautions for Tepylute
Myelosuppression
For patients receiving TEPYLUTE for treatment of adenocarcinoma of the breast or adenocarcinoma of the ovary, if the bone marrow has been compromised by prior irradiation or chemotherapy, or is recovering from chemotherapy, the risk of severe myelosuppression with TEPYLUTE may be increased. Perform periodic complete blood counts during the course of treatment with TEPYLUTE. Provide supportive care for infections, bleeding, and symptomatic anemia [see ADVERSE REACTIONS].
Hypersensitivity
Clinically significant hypersensitivity reactions, including anaphylaxis, have occurred following administration of thiotepa. If anaphylactic or other clinically significant allergic reaction occurs, discontinue treatment with TEPYLUTE, initiate appropriate therapy, and monitor until signs and symptoms resolve [see CONTRAINDICATIONS and ADVERSE REACTIONS].
Cutaneous Toxicity
TEPYLUTE and/or its active metabolites may be excreted in part via skin in patients receiving high-dose therapy. Treatment with TEPYLUTE may cause skin discoloration, pruritus, blistering, desquamation, and peeling that may be more severe in the groin, axillae, skin folds, in the neck area, and under dressings. Instruct patients to shower or bathe with water at least twice daily through 48 hours after administration of TEPYLUTE. Change occlusive dressing and clean the covered skin at least twice daily through 48 hours after administration of TEPYLUTE. Change bed sheets daily during treatment.
Skin reactions associated with accidental exposure to TEPYLUTE may occur. Wash the skin thoroughly with soap and water in case TEPYLUTE solution contacts the skin. Flush mucous membranes in case of TEPYLUTE contact with mucous membranes.
Concomitant Use Of Live And Attenuated Vaccines
Do not administer live or attenuated viral or bacterial vaccines to a patient treated with TEPYLUTE until the immunosuppressive effects have resolved.
Hepatic Veno-Occlusive Disease
Monitor by physical examination, serum transaminases and bilirubin, and provide supportive care to patients who develop hepatic veno-occlusive disease.
Central Nervous System Toxicity
Fatal encephalopathy has occurred in patients treated with high doses of thiotepa. Other central nervous system toxicities, such as headache, apathy, psychomotor retardation, disorientation, confusion, amnesia, hallucinations, drowsiness, somnolence, seizures, coma, inappropriate behavior and forgetfulness have been reported to occur in a dose-dependent manner during or shortly after administration of high-dose thiotepa. Do not exceed the recommended dose of TEPYLUTE. If severe or life-threatening central nervous system toxicity occurs, discontinue administration of TEPYLUTE and provide supportive care.
Carcinogenicity
Like many alkylating agents, thiotepa has been reported to be carcinogenic when administered to laboratory animals [see Nonclinical Toxicity]. Carcinogenicity is shown most clearly in studies using mice, but there is some evidence of carcinogenicity in man. There is an increased risk of a secondary malignancy with use of TEPYLUTE.
Polyethylene Glycol 400 Toxicity
TEPYLUTE contains a high concentration of polyethylene glycol (PEG) 400. Based on findings in animals, administration of high amounts of PEG 400 may cause damage to the kidneys and liver at dosages higher than recommended. When prescribing TEPYLUTE, take into consideration the PEG 400 load from concomitant medications.
Embryo-Fetal Toxicity
Based on the mechanism of action and findings in animals, TEPYLUTE can cause fetal harm when administered to a pregnant woman. There are no adequate and well-controlled studies of TEPYLUTE in pregnant women. Thiotepa given by the intraperitoneal (IP) route was teratogenic in mice at doses ≥ 1 mg/kg (3.2 mg/m2), approximately 8-fold less than the maximum recommended human therapeutic dose (0.8 mg/kg, 27 mg/m2), based on body-surface area. Thiotepa given by the IP route was teratogenic in rats at doses ≥ 3 mg/kg (21 mg/m2), approximately equal to the maximum recommended human therapeutic dose, based on body-surface area. Thiotepa was lethal to rabbit fetuses at a dose of 3 mg/kg (41 mg/m2), approximately two times the maximum recommended human therapeutic dose based on body-surface area.
Advise pregnant women of the potential risk to the fetus [see Use In Specific Populations]. Advise females of reproductive potential to use highly effective contraception during and after treatment with TEPYLUTE for 6 months after therapy. Advise males of reproductive potential to use effective contraception during and after treatment with TEPYLUTE for 1 year after therapy [see Use In Specific Populations].
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment Of Fertility
In mice, repeated intraperitoneal (IP) administration of thiotepa (1.15 or 2.3 mg/kg three times per week for 52 or 43 weeks, respectively) produced a significant increase in the combined incidence of squamous-cell carcinomas of the skin, preputial gland, and ear canal, and combined incidence of lymphoma and lymphocytic leukemia. In other studies in mice, repeated IP administration of thiotepa (4 or 8 mg/kg three times per week for 4 weeks followed by a 20 week observation period or 1.8 mg/kg three times per week for 4 weeks followed by a 35 week observation period) resulted in an increased incidence of lung tumors. In rats, repeated IP administration of thiotepa (0.7 or 1.4 mg/kg three times per week for 52 or 34 weeks, respectively) produced significant increases in the incidence of squamous-cell carcinomas of the skin or ear canal, combined hematopoietic neoplasms, and uterine adenocarcinomas. Thiotepa given intravenously (IV) to rats (1 mg/kg once per week for 52 weeks) produced an increased incidence of malignant tumors (abdominal cavity sarcoma, lymphosarcoma myelosis, seminoma, fibrosarcoma, salivary gland hemangioendothelioma, mammary sarcoma, pheochromocytoma) and benign tumors.
The lowest reported carcinogenic dose in mice (1.15 mg/kg, 3.68 mg/m2) is approximately 7-fold less than the maximum recommended human therapeutic dose based on body-surface area. The lowest reported carcinogenic dose in rats (0.7 mg/kg, 4.9 mg/m2) is approximately 6-fold less than the maximum recommended human therapeutic dose based on body-surface area.
Thiotepa was mutagenic in in vitro assays in Salmonella typhimurium, E coli, Chinese hamster lung and human lymphocytes. Chromosomal aberrations and sister chromatid exchanges were observed in vitro with thiotepa in bean root tips, human lymphocytes, Chinese hamster lung, and monkey lymphocytes.
Mutations were observed with oral thiotepa in mouse at doses > 2.5 mg/kg (8 mg/m2). The mouse micronucleus test was positive with intraperitoneal administration of > 1 mg/kg (3.2 mg/m2). Other positive in vivo chromosomal aberration or mutation assays included Drosophila melanogaster, Chinese hamster marrow, murine marrow, monkey lymphocyte, and murine germ cell.
Thiotepa impaired fertility in male mice at oral or intraperitoneal doses ≥ 0.7 mg/kg (2.24 mg/m2), approximately 12-fold less than the maximum recommended human therapeutic dose based on body-surface area. Thiotepa (0.5 mg) inhibited implantation in female rats when instilled into the uterine cavity. Thiotepa interfered with spermatogenesis in mice at IP doses ≥ 0.5 mg/kg (1.6 mg/m2), approximately 17-fold less than the maximum recommended human therapeutic dose based on body-surface area. Thiotepa interfered with spermatogenesis in hamsters at an IP dose of 1 mg/kg (4.1 mg/m2), approximately 7-fold less than the maximum recommended human therapeutic dose based on body-surface area.
Use In Specific Populations
Pregnancy
Risk Summary
TEPYLUTE can cause fetal harm when administered to a pregnant woman based on findings from animals and the drug’s mechanism of action [see CLINICAL PHARMACOLOGY]. Limited available data with thiotepa use in pregnant women are insufficient to inform a drug-associated risk of major birth defects and miscarriage. In animal reproduction studies, administration of thiotepa to pregnant mice and rats during organogenesis produced teratogenic effects (neural tube defects and malformations of the skeletal system of the fetus) at doses approximately 0.125 and 1 times, respectively, the maximum recommended human daily dose on a mg/m2 basis. Thiotepa was lethal to rabbit fetuses at approximately 2 times the maximum recommended human therapeutic dose based on body-surface area [see Data]. Consider the benefits and risks of TEPYLUTE for the mother and possible risks to the fetus when prescribing TEPYLUTE to a pregnant woman.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Thiotepa given by the IP route in mice at doses ≥ 1 mg/kg (3.2 mg/m2), approximately 8-fold less than the maximum recommended human therapeutic dose based on body-surface area, and in rats at doses ≥ 3 mg/kg (21 mg/m2), approximately equal to the maximum recommended human therapeutic dose based on body-surface area, resulted in various malformations including neural tube defects, omphalocele, renal agenesis, atresia ani, limb and digit defects, cleft palate, micrognathia, other skeletal anomalies in the skull, vertebrae and ribs, and reduced skeletal ossification. Thiotepa was lethal to rabbit fetuses at a dose of 3 mg/kg (41 mg/m2), approximately 2 times the maximum recommended human therapeutic dose based on body-surface area.
Lactation
Risk Summary
There is no information regarding the presence of thiotepa in human milk, the effects on the breastfed infant, or the effects on milk production.
Because of the potential for serious adverse reactions, including the potential for tumorigenicity shown for thiotepa in animal studies, advise patients not to breastfeed during TEPYLUTE treatment and for 1 week after the last dose.
Females And Males Of Reproductive Potential
TEPYLUTE can cause fetal harm when administered to a pregnant woman [see Pregnancy].
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating TEPYLUTE therapy.
Contraception
Females
Advise females of reproductive potential to avoid pregnancy during TEPYLUTE treatment and for 6 months after the last dose of TEPYLUTE. Advise females to immediately report pregnancy [see Pregnancy].
Males
TEPYLUTE may damage spermatozoa and testicular tissue, resulting in possible genetic abnormalities. Males with female sexual partners of reproductive potential should use effective contraception during TEPYLUTE treatment and for 1 year after the last dose of TEPYLUTE [see Nonclinical Toxicology].
Infertility
Based on nonclinical findings, male and female fertility may be compromised by treatment with TEPYLUTE. Inform male patients about the possibility of sperm conservation before the start of therapy [see Nonclinical Toxicology].
Pediatric Use
Safety and effectiveness of TEPYLUTE in neonates have not been established.
Safety and effectiveness of TEPYLUTE for treatment of adenocarcinoma of the breast and adenocarcinoma of the ovary in pediatric patients have not been established.
Geriatric Use
Clinical studies of thiotepa for treatment of adenocarcinoma of the breast and adenocarcinoma of the ovary did not include sufficient numbers of subjects aged 65 and over to determine whether elderly subjects respond differently from younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreasing hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
Renal Impairment
In patients with moderate (creatinine clearance (CLcr) of 30 mL/min to 59 mL/min) renal impairment, decreased renal excretion may result in increased plasma levels of thiotepa and TEPA [see CLINICAL PHARMACOLOGY]. This may result in increased toxicity. Monitor patients with moderate to severe (CLcr < 30 mL/min) renal impairment for signs and symptoms of toxicity following treatment with TEPYLUTE for an extended period of time.
Hepatic Impairment
Thiotepa is extensively metabolized in the liver. Patients with moderate (bilirubin levels greater than 1.5 times to 3 times the upper limit of normal and any AST) hepatic impairment may have increased plasma levels of thiotepa [see CLINICAL PHARMACOLOGY]. This may result in toxicity. Monitor patients with moderate to severe (bilirubin levels greater than 3 times upper limit of normal and any AST) hepatic impairment for signs and symptoms of toxicity following treatment with TEPYLUTE for an extended period of time.
Overdose Information for Tepylute
There is no experience with overdoses of thiotepa. The most important adverse reactions expected in case of overdose are myeloablation and pancytopenia [see Nonclinical Toxicology]. There is no known antidote for thiotepa. Monitor the hematological status closely and provide vigorous supportive measures as medically indicated.
Contraindications for Tepylute
TEPYLUTE is contraindicated in:
- Patients with severe hypersensitivity to thiotepa [see WARNINGS AND PRECAUTIONS]
- Concomitant use with live or attenuated vaccines [see WARNINGS AND PRECAUTIONS]
Clinical Pharmacology for Tepylute
Mechanism Of Action
Thiotepa is an alkylating drug of the polyfunctional type, related chemically and pharmacologically to the nitrogen mustard. The radiomimetic action of thiotepa is believed to occur through the release of ethyleneimine radicals which, like irradiation, disrupt the bonds of DNA. One of the principle bond disruptions is initiated by alkylation of guanine at the N-7 position, which severs the linkage between the purine base and the sugar and liberates alkylated guanines.
Pharmacokinetics
Absorption
Thiotepa reached maximal concentrations close to the end of infusion following an intravenous infusion.
Distribution
The binding of thiotepa to plasma proteins is approximately 10% to 20%. In adults administered intravenous thiotepa between 20 mg to 250 mg/m2 as an intravenous bolus or infusion up to 4 hours, the mean volume of distribution of thiotepa ranged from 1.0 L/kg (30%) to 1.9 L/kg (17%).
Elimination
In adults administered intravenous thiotepa between 20 mg to 250 mg/m2 as an intravenous bolus or infusion up to 4 hours, the mean thiotepa clearance ranged from 14.6 L/hr/m2 (23%) to 27.9 L/hr/m2 (69%). In adult population, the mean terminal elimination half-life ranged from 1.4 hours (7%) to 3.7 hours (14%) for thiotepa and from 4.9 hours to 17.6 hours (20%) for TEPA.
Metabolism
Thiotepa undergoes hepatic metabolism. In vitro data suggests that CYP3A4 and CYP2B6 may be responsible for the metabolism of thiotepa to TEPA, a major active metabolite.
Excretion
In adult patients, urinary excretion of thiotepa accounted for less than 2% of the dose and TEPA accounted for 11% or less of the dose.
Specific Populations
Hepatic Impairment
The exposure (as measured by area under the curve (AUC)) of thiotepa increased by 1.6-fold and 1.8-fold following administration of multiple thiotepa doses of 7 mg/kg administered every 2 days with cyclophosphamide in two adult patients who had liver metastases with moderate hepatic impairment compared to the exposure observed in one patient with normal hepatic function. The effect of severe hepatic impairment on thiotepa exposure is unknown.
Renal Impairment
The exposure (as measured by AUC) of thiotepa increased by 1.4-fold and TEPA increased by 2.6-fold following administration of multiple doses of 120 mg/m2/day in one patient with moderate renal impairment (CLcr = 38 mL/min) administered cyclophosphamide plus thiotepa plus carboplatin, compared to exposure of thiotepa in patients with normal renal function. The effects of severe renal impairment or end-stage renal disease on thiotepa exposure are unknown.
Drug Interactions
The clinical relevance of in vitro inhibition of the cytochrome P450 enzymes described below is unknown, but it cannot be excluded that the systemic exposure of thiotepa or medicinal products that are substrates for these enzymes may be affected with concomitant administration with TEPYLUTE.
Effect Of Cytochrome P450 Modulators On Thiotepa
In vitro data demonstrates that CYP3A4 and CYP2B6 inhibitors decrease the metabolism of thiotepa [see DRUG INTERACTIONS].
Effect Of Thiotepa On Cytochrome P450 2B6
In vitro data demonstrates that thiotepa inhibits CYP2B6.
Effect Of Thiotepa On Cyclophosphamide
The administration of thiotepa 1.5 hours prior to intravenous cyclophosphamide in patients administered cyclophosphamide plus thiotepa plus carboplatin decreased the AUC of 4-hydroxycyclophosphamide by 26% and maximal concentrations of 4-hydroxycyclophosphamide by 62%, compared to administration of cyclophosphamide prior to thiotepa.
Patient Information for Tepylute
Hypersensitivity
Counsel patients on the signs and symptoms of hypersensitivity and to seek immediate emergency assistance if they develop any of these signs and symptoms [see WARNINGS AND PRECAUTIONS].
Myelosuppression
Inform patients of the possibility of developing low blood cell counts and the need for hematopoietic progenitor cell infusion. Instruct patients to immediately report to their healthcare provider if bleeding or fever occurs [see WARNINGS AND PRECAUTIONS].
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to inform their healthcare provider if they are pregnant or become pregnant.
Advise females of reproductive potential to use effective contraception during treatment with TEPYLUTE and for 6 months after the last dose [see WARNINGS AND PRECAUTIONS, Use In Specific Populations].
Advise males with female partners of reproductive potential to use effective contraception during TEPYLUTE treatment and for 1 year after the last dose of TEPYLUTE [see Use In Specific Populations].
Advise patients that TEPYLUTE can produce infertility. Inform male patients about the possibility of sperm conservation before the start of therapy [see Use In Specific Populations].
Lactation
Advise women not to breastfeed while receiving TEPYLUTE and for 1 week after the last dose [see Use In Specific Populations].
Secondary Malignancies
Inform patients that TEPYLUTE can increase the risk of secondary malignancy [see WARNINGS AND PRECAUTIONS].
From 

Report Problems to the Food and Drug Administration
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit the FDA MedWatch website or call 1-800-FDA-1088.